Glucose
About this schools Wikipedia selection
This content from Wikipedia has been selected by SOS Children for suitability in schools around the world. Do you want to know about sponsoring? See www.sponsorachild.org.uk
| D-Glucose | |
|---|---|
 |
|
 Glucose C6H12O6
|
|
|
Preferred IUPAC name
D-glucose |
|
|
(2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanal |
|
|
Other names
Blood sugar |
|
| Identifiers | |
| Abbreviations | Glc |
| CAS number | 50-99-7 |
| PubChem | 5793 |
| ChemSpider | 5589 |
| UNII | 5SL0G7R0OK |
| EC number | 200-075-1 |
| KEGG | C00031 |
| MeSH | Glucose |
| ChEBI | CHEBI:4167 |
| ChEMBL | CHEMBL1222250 |
| RTECS number | LZ6600000 |
| ATC code | B05, V04 CA02, V06 DC01 |
| Beilstein Reference | 1281604 |
| Gmelin Reference | 83256 |
| 3DMet | B04623 |
| Jmol-3D images | Image 1 Image 2 |
|
SMILES
|
|
|
InChI
|
|
| Properties | |
| Molecular formula | C6H12O6 |
| Molar mass | 180.16 g mol−1 |
| Appearance | White powder |
| Density | 1.54 g/cm3 |
| Melting point |
α-D-glucose: 146 °C |
| Solubility in water | 91 g/100 mL |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−1271 kJ/mol |
| Std enthalpy of combustion ΔcH |
−2805 kJ/mol |
| Standard molar entropy S |
209.2 J K−1 mol−1 |
| Specific heat capacity, C | |
| Hazards | |
| MSDS | ICSC 0865 |
| EU Index | not listed |
| NFPA 704 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Glucose ( / ˈ ɡ l uː k oʊ s / or / - k oʊ z /; C6H12O6, also known as D-glucose, dextrose, or grape sugar) is a simple monosaccharide found in plants. It is one of the three dietary monosaccharides, along with fructose and galactose, that are absorbed directly into the bloodstream during digestion. An important carbohydrate in biology, cells use it as the primary source of energy and a metabolic intermediate. Glucose is one of the main products of photosynthesis and fuels for cellular respiration. Glucose exists in several different molecular structures, but all of these structures can be divided into two families of mirror-images ( stereoisomers). Only one set of these isomers exists in nature, those derived from the " right-handed form" of glucose, denoted D-glucose. D-glucose is sometimes referred to as dextrose, although the use of this name is strongly discouraged. The term dextrose is derived from dextrorotatory glucose. This name is therefore confusing when applied to the enantiomer, which rotates light in the opposite direction. Starch and cellulose are polymers derived from the dehydration of D-glucose. The other stereoisomer, called L-glucose, is hardly ever found in nature.
The name "glucose" comes from the Greek word glukus (γλυκύς), meaning "sweet". The suffix " -ose" denotes a sugar.
Function
Why glucose—and not another monosaccharide such as fructose—is so widely used in organisms is not clearly understood. One reason might be that glucose has a lower tendency, relative to other hexose sugars, to react non-specifically with the amino groups of proteins. This reaction ( glycation) reduces or destroys the function of many enzymes. The low rate of glycation is due to glucose's preference for the less reactive cyclic isomer. Nevertheless, many of the long-term complications of diabetes (e.g., blindness, renal failure, and peripheral neuropathy) are probably due to the glycation of proteins or lipids. In contrast, enzyme-regulated addition of glucose to proteins by glycosylation is often essential to their function. Another reason as to why glucose is the most common sugar is that it is the most conformationally stable among other possibilities.
Analyte in medical blood test
Glucose is a common medical analyte measured in blood samples. Eating or fasting prior to taking a blood sample has an effect on the result. A high fasting glucose blood sugar level may be a sign of prediabetes or diabetes mellitus.
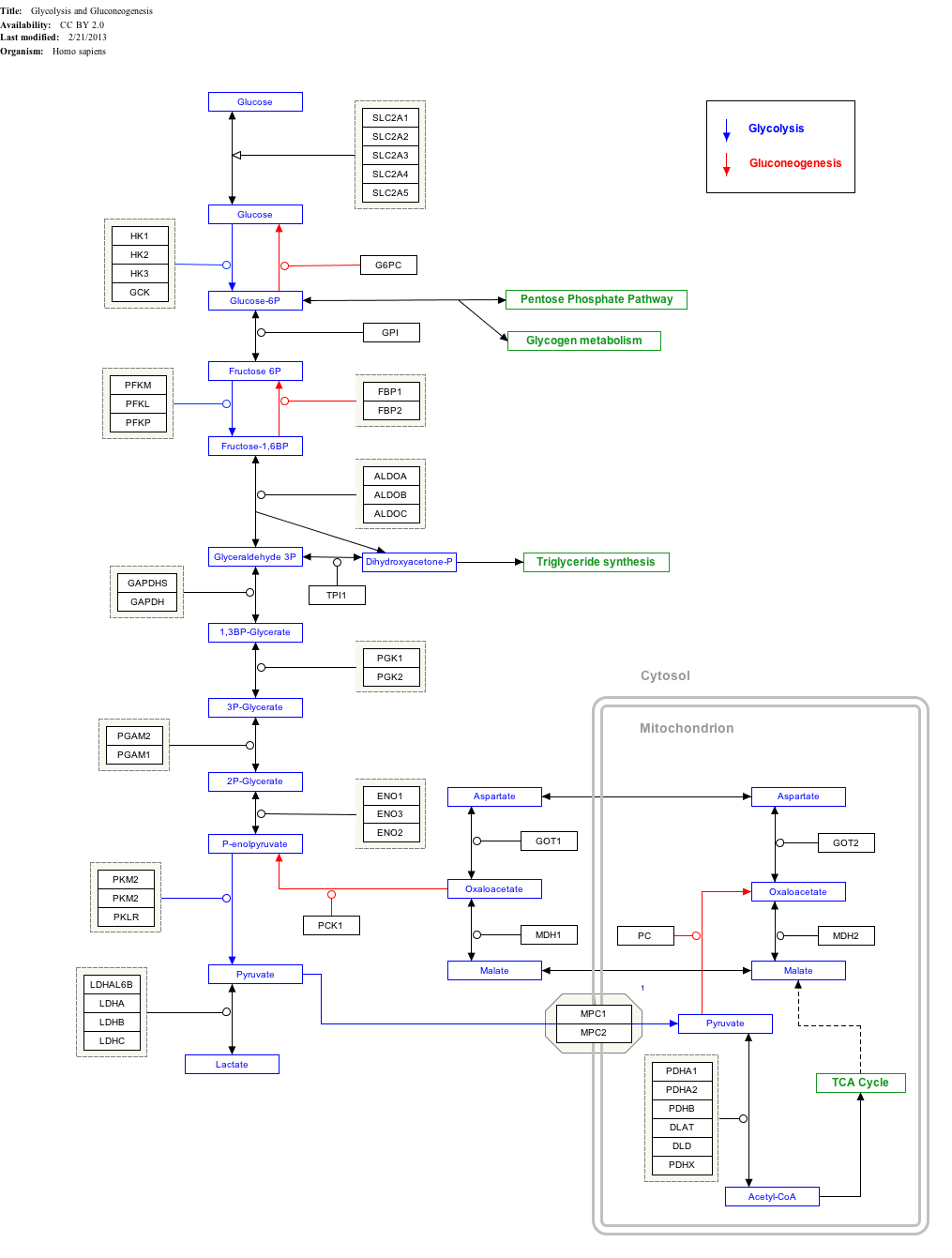
Glycolysis
|
||||||||||||||||||||
| Compound C00031 at KEGG Pathway Database. Enzyme 2.7.1.1 at KEGG Pathway Database. Compound C00668 at KEGG Pathway Database. Reaction R01786 at KEGG Pathway Database. | ||||||||||||||||||||

-Glucose-containing compounds and isomeric forms are digested and taken up by the body in the intestines, including starch, glycogen, disaccharides and monosaccharides.
-Glucose is stored in mainly the liver and muscles as glycogen.
-It is distributed and utilized in tissues as free glucose.
Use of glucose as an energy source in cells is via aerobic or anaerobic respiration. Both of these start with the early steps of the glycolysis metabolic pathway. The first step of this is the phosphorylation of glucose by hexokinase to prepare it for later breakdown to provide energy. The major reason for the immediate phosphorylation of glucose by a hexokinase is to prevent diffusion out of the cell. The phosphorylation adds a charged phosphate group so the glucose 6-phosphate cannot easily cross the cell membrane. Irreversible first steps of a metabolic pathway are common for regulatory purposes.
In anaerobic respiration one glucose molecule produces a net gain of two ATP molecules (four ATP molecules are produced during glycolysis but two are required by enzymes used during the process). In aerobic respiration a molecule of glucose is much more profitable in that a net worth of 32 ATP molecules is generated (34 gross with two being required in the process).
Click on genes, proteins and metabolites below to link to respective articles.
- ^ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
Precursor
Organisms use glucose as a precursor for the synthesis of several important substances. Starch, cellulose, and glycogen ("animal starch") are common glucose polymers ( polysaccharides). Some of these polymers like starch or glycogen serve as energy stores while others like cellulose and chitin (which is made from a derivative of glucose) have structural roles. Oligosaccharides of glucose combined with other sugars serve as important energy stores. These include lactose, the predominant sugar in milk which a glucose-galactose disaccharide and sucrose, another disaccharide of glucose and fructose. Glucose is also added onto certain proteins and lipids in a process called glycosylation. This is often critical for their functioning. The enzymes that join glucose to other molecules usually use phosphorylated glucose to power the formation of the new bond by breaking the glucose-phosphate bond.
Other than its direct use as a monomer, glucose can be broken down to synthesize a wide variety of other biomolecules. This is important as glucose serves both as a primary store of energy but also as a source of organic carbon. Glucose can be broken down and converted into lipids. It is also a precursor for the synthesis of other important molecules like vitamin C ( ascorbic acid). Though plants and some microbes can create all the compounds they need from glucose given the necessary minerals, all animals and many microbes cannot synthesize some or
Structure and nomenclature
Glucose is a monosaccharide with formula C6H12O6 or H-(C=O)-(CHOH)5-H, whose five hydroxyl (OH) groups are arranged in a specific way along its six-carbon backbone.
Open-chain form
In its fleeting open-chain form, the glucose molecule has an open (as opposed to cyclic) and unbranched backbone of six carbon atoms, C-1 through C-6; where C-1 is part of an aldehyde group H(C=O)-, and each of the other five carbons bears one hydroxyl group -OH. The remaining bonds of the backbone carbons are satisfied by hydrogen atoms -H. Therefore glucose is a hexose and an aldose, or an aldohexose.
Each of the four carbons C-2 through C-5 is a stereocenter, meaning that its four bonds connect to four different substitutents. (Carbon C-2, for example, connects to -(C=O)H, -OH, -H, and -(CHOH)4H.) In D-glucose, these four parts must be in a specific three-dimensional arrangement. Namely, when the molecule is drawn in the Fischer projection, the hydroxyls on C-2, C-4, and C-5 must be on the right side, while that on C-3 must be on the left side.
The positions of those four hydroxyls are exactly reversed in the Fischer diagram of L-glucose. D- and L-glucose are two of the 16 possible aldohexoses; the other 14 are allose, altrose, mannose, gulose, idose, galactose, and talose, each with two enantiomers, "D-" and "L-".
Cyclic forms
In solutions, the open-chain form of glucose (either "D-" or "L-") exists in equilibrium with several cyclic isomers, each containing a ring of carbons closed by one oxygen atom. In aqueous solution however, more than 99% of glucose molecules, at any given time, exist as pyranose. The open-chain form is limited to about 0.25% and furanose exists in negligible amounts. The terms "glucose" and "D-glucose" are generally used for these cyclic forms as well. The ring arises from the open-chain form by a nucleophilic addition reaction between the aldehyde group -(C=O)H at C-1 and the hydroxyl group -OH at C-4 or C-5, yielding a hemiacetal group -C(OH)H-O-.
The reaction between C-1 and C-5 creates a molecule with a six-membered ring, called pyranose, after the cyclic ether pyran, the simplest molecule with the same carbon-oxygen ring. The (much rarer) reaction between C-1 and C-4 creates a molecule with a five-membered ring, called furanose, after the cyclic ether furan. In either case, each carbon in the ring has one hydrogen and one hydroxyl attached, except for the last carbon (C-4 or C-5) where the hydroxyl is replaced by the remainder of the open molecule (which is -(C(CH2OH)HOH)-H or -(CHOH)-H, respectively).
The ring-closing reaction makes carbon C-1 chiral, too, since its four bonds lead to -H, to -OH, to carbon C-2, and to the ring oxygen. These four parts of the molecule may be arranged around C-1 (the anomeric carbon) in two distinct ways, designated by the prefixes "α-" and "β-". When a glucopyranose molecule is drawn in the Haworth projection, the designation "α-" means that the hydroxyl group attached to C-1 and the -CH2OH group at C-5 lies on opposite sides of the ring's plane (a trans arrangement), while "β-" means that they are on the same side of the plane (a cis arrangement).
Therefore, the open-chain isomer D-glucose gives rise to four distinct cyclic isomers: α-D-glucopyranose, β-D-glucopyranose, α-D-glucofuranose, and β-D-glucofuranose; which are all chiral.
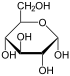 α-D- |
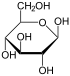 β-D- |
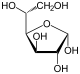 α-D- |
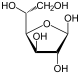 β-D- |
The other open-chain isomer L-glucose similarly gives rise to four distinct cyclic forms of L-glucose, each the mirror image of the corresponding D-glucose.
The rings are not planar but twisted in three dimensions. The glucopyranose ring (α or β) can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of cyclohexane. Similarly, the glucofuranose ring may assume several shapes, analogous to the "envelope" conformations of cyclopentane.
The glucopyranose forms of glucose predominate in solution, and are the only forms observed in the solid state. They are crystalline colorless solids, highly soluble in water and acetic acid, poorly soluble in methanol and ethanol. They melt at 146 °C (295 °F) (α) and 150 °C (302 °F) (β), and decompose at higher temperatures into carbon and water.
Rotational isomers
Each glucose isomer is subject to rotational isomerism. Within the cyclic form of glucose, rotation may occur around the O6-C6-C5-O5 torsion angle, termed the ω-angle, to form three staggered rotamer conformations called gauche-gauche (gg), gauche-trans (gt) and trans-gauche (tg). For methyl α-D-glucopyranose at equilibrium the ratio of molecules in each rotamer conformation is reported as 57:38:5 gg:gt:tg. This tendency for the ω-angle to prefer to adopt a gauche conformation is attributed to the gauche effect.
Physical properties
Solutions
All forms of glucose are colorless and easily soluble in water, acetic acid, and several other solvents. They are only sparingly soluble in methanol and ethanol.
The open-chain form is thermodynamically unstable, and it spontaneously isomerizes to the cyclic forms. (Although the ring closure reaction could in theory create four- or three-atom rings, these would be highly strained and are not observed.) In solutions at room temperature, the four cyclic isomers interconvert over a time scale of hours, in a process called mutarotation. Starting from any proportions, the mixture converges stable ratio of α:β 36:64. The ratio would be α:β 11:89 if it were not for the influence of the anomeric effect. Mutarotation is considerably slower at temperatures close to 0 °C.
Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a re-forming of the ring. The ring closure step may use a different -OH group than the one recreated by the opening step (thus switching between pyranose and furanose forms), and/or the new hemiacetal group created on C-1 may have the same or opposite handedness as the original one (thus switching between the α and β forms). Thus, even though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
Solid state
Depending on conditions, three major solid forms of glucose can be crystallised from water solutions: α-glucopyranose, β-glucopyranose, and β-glucopyranose hydrate.
Optical activity
Whether in water or in the solid form, D-glucose is dextrorotatory, meaning that it will rotate the direction of polarized light clockwise. The effect is due to the chirality of the molecules, and indeed the mirror-image isomer, L-glucose, is levorotatory (rotates polarized light counterclockwise) by the same amount. The strength of the effect is different for each of the five tautomers.
Note that the D- prefix does not refer directly to the optical properties of the compound. It indicates that the C-2 chiral centre has the same handedness as that of D-glyceraldehyde (which was so labeled because it is dextrorotatory). The fact that D-glucose is dextrorotatory is a combined effect of its four chiral centers, not just of C-2; and indeed some of the other D-aldohexoses are levorotatory.
Production
| Metabolism of common monosaccharides and some biochemical reactions of glucose |
|---|
Biosynthesis
In plants and some prokaryotes, glucose is a product of photosynthesis. In animals and fungi, glucose results from the breakdown of glycogen, a process known as glycogenolysis. In plants the breakdown substrate is starch.
In animals, glucose is synthesized in the liver and kidneys from non-carbohydrate intermediates, such as pyruvate, lactate and glycerol, by a process known as gluconeogenesis.
In some deep-sea bacteria, glucose is produced by chemosynthesis.
Commercial
Glucose is produced commercially via the enzymatic hydrolysis of starch. Many crops can be used as the source of starch. Maize, rice, wheat, cassava, corn husk and sago are all used in various parts of the world. In the United States, cornstarch (from maize) is used almost exclusively. Most commercial glucose occurs as a component of invert sugar, an approximately 1:1 mixture of glucose and fructose. In principle, cellulose could be hydrolysed to glucose, but this process is not yet commercially practical.
History
Because glucose is a basic necessity of many organisms, a correct understanding of its chemical makeup and structure contributed greatly to a general advancement in organic chemistry. This understanding occurred largely as a result of the investigations of Emil Fischer, a German chemist who received the 1902 Nobel Prize in Chemistry as a result of his findings. The synthesis of glucose established the structure of organic material and consequently formed the first definitive validation of Jacobus Henricus van't Hoff's theories of chemical kinetics and the arrangements of chemical bonds in carbon-bearing molecules. Between 1891 and 1894, Fischer established the stereochemical configuration of all the known sugars and correctly predicted the possible isomers, applying van't Hoff's theory of asymmetrical carbon atoms.