Photosynthetic reaction centre
Did you know...
This Schools selection was originally chosen by SOS Children for schools in the developing world without internet access. It is available as a intranet download. SOS mothers each look after a a family of sponsored children.
A photosynthetic reaction centre (or photosynthetic reaction centre) is a complex of several proteins, pigments and other co-factors assembled together to execute the primary energy conversion reactions of photosynthesis. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer reactions along a series of protein-bound co-factors. These co-factors are light-absorbing molecules (also named chromophores or pigments) such as chlorophyll and phaeophytin, as well as quinones. The energy of the photon is used to promote an electron to a higher molecular energy level of a pigment. The free energy created is then used to reduce a chain of nearby electron acceptors, which have subsequently higher redox-potentials. These electron transfer steps are the initial phase of a series of energy conversion reactions, ultimately resulting in the production of chemical energy during photosynthesis.
Transforming light energy into charge separation
Reaction centres are present in all green plants, algae, and many bacteria. Although these species are separated by billions of years of evolution, the reaction centres are homologous for all photosynthetic species. In contrast, a large variety in light-harvesting complexes exist between the photosynthetic species. Green plants and algae have two different types of reaction centres that are part of larger supercomplexes known as photosystem IP700 and photosystem II P680. The structures of these supercomplexes are large, involving multiple light-harvesting complexes. The reaction centre found in Rhodopseudomonas bacteria is currently best understood, since it was the first reaction centre of known structure and has fewer polypeptide chains than the examples in green plants.
A reaction centre is laid out in such a way that it captures the energy of a photon using pigment molecules and turns it into a usable form. Once the light energy has been absorbed directly by the pigment molecules, or passed to them by resonance transfer from a surrounding light-harvesting complex, they release two electrons into an electron transport chain.
Light is made up of small bundles of energy called photons. If a photon with the right amount of energy hits an electron, it will raise the electron to a higher energy level. Electrons are most stable at their lowest energy level, what is also called its ground state. In this state, the electron is in the orbit that has the least amount of energy. Electrons in higher energy levels can return to ground state in a manner analogous to a ball falling down a staircase. In doing so, the electrons release energy. This is the process that is exploited by a photosynthetic reaction centre.
When an electron rises to a higher energy level, decrease in the reduction potential of the molecule in which the electron resides occurs. This means that the molecule has a greater tendency to donate electrons, the key to the conversion of light energy to chemical energy. In green plants, the electron transport chain that follows has many electron acceptors including phaeophytin, quinone, plastoquinone, cytochrome bf, and ferredoxin, which result in the reduced molecule NADPH. The passage of the electron through the electron transport chain also results in the pumping of protons (hydrogen ions) from the chloroplast's stroma into the lumen, resulting in a proton gradient across the thylakoid membrane that can be used to synthesise ATP using ATP synthase. Both the ATP and NADPH are used in the Calvin cycle to fix carbon dioxide into triose sugars.
Bacteria
Structure
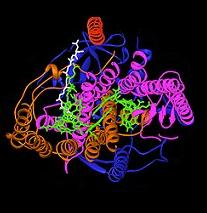
The bacterial photosynthetic reaction centre has been an important model to understand the structure and chemistry of the biological process of capturing light energy. In the 1960s, Roderick Clayton was the first to purify the reaction centre complex from purple bacteria. However, the first crystal structure was determined in 1982 by Hartmut Michel, Johann Deisenhofer and Robert Huber for which they shared the Nobel Prize in 1988. This was also significant, since it was the first structure for any membrane protein complex.
Four different subunits were found to be important for the function of the photosynthetic reaction centre. The L and M subunits, shown in blue and purple in the image of the structure, both span the lipid bilayer of the plasma membrane. They are structurally similar to one another, both having 5 transmembrane alpha helices. Four bacteriochlorophyll b (BChl-b) molecules, two bacteriophaeophytin b molecules (BPh) molecules, two quinones (QA and QB), and a ferrous ion are associated with the L and M subunits. The H subunit, shown in gold, lies on the cytoplasmic side of the plasma membrane. A cytochrome subunit, here not shown, contains four c-type haems and is located on the periplasmic surface (outer) of the membrane. The latter sub-unit is not a general structural motif in photosynthetic bacteria. The L and M subunits bind the functional and light-interacting cofactors, shown here in green.
Reaction centres from different bacterial species may contain slightly altered bacterio-chlorophyll and bacterio-phaeophytin chromophores as functional co-factors. These alterations cause shifts in the colour of light that can be absorbed, thus creating specific niches for photosynthesis. The reaction centre contains two pigments that serve to collect and transfer the energy from photon absorption: BChl and Bph. BChl roughly resembles the chlorophyll molecule found in green plants, but, due to minor structural differences, its peak absorption wavelength is shifted into the infrared, with wavelengths as long as 1000 nm. Bph has the same structure as BChl, but the central magnesium ion is replaced by two protons. This alteration causes both an absorbance maximum shift and a lowered redox-potential.
Mechanism
The process starts when light is absorbed by two BChl molecules (a dimer) that lie near the periplasmic side of the membrane. This pair of chlorophyll molecules, often called the "special pair", absorbs photons between 870 nm and 960 nm, depending on the species and, thus, is called P870 (for the species rhodobacter sphaeroides) or P960 (for rhodopseudomonas viridis), with P standing for "pigment"). Once P absorbs a photon, it ejects an electron, which is transferred through another molecule of Bchl to the BPh in the L subunit. This initial charge separation yields a positive charge on P and a negative charge on the BPh. This process takes place in 10 picoseconds (10−11 seconds).
The charges on the specialpair + and the BPh- could undergo charge recombination in this state. This would waste the high-energy electron and convert the absorbed light energy in to heat. Several factors of the reaction centre structure serve to prevent this. First, the transfer of an electron from BPh- to P960+ is relatively slow compared to two other redox reactions in the reaction centre. The faster reactions involve the transfer of an electron from BPh- (BPh- is oxidised to BPh) to the electron acceptor quinone (QA), and the transfer of an electron to P960+ (P960+ is reduced to P960) from a haem in the cytochrome subunit above the reaction centre.
The high-energy electron that resides on the tightly bound quinone molecule QA is transferred to an exchangeable quinone molecule QB. This molecule is loosely associated with the protein and is fairly easy to detach. Two of the high-energy electrons are required to fully reduce QB to QH2, taking up two protons from the cytoplasm in the process. The reduced quinone QH2 diffuses through the membrane to another protein complex ( cytochrome bc1-complex) where it is oxidised. In the process the reducing power of the QH2 is used to pump protons across the membrane to the periplasmic space. The electrons from the cytochrome bc1-complex are then transferred through a soluble cytochrome c intermediate, called cytochrome c2, in the periplasm to the cytochrome subunit. Thus, the flow of electrons in this system is cyclical.
Green plants
Oxygenic photosynthesis
In 1772, the chemist Joseph Priestley carried out a series of experiments relating to the gases involved in respiration and combustion. In his first experiment, he lit a candle and placed it under an upturned jar. After a short period of time, the candle burned out. He carried out a similar experiment with a mouse in the confined space of the burning candle. He found that the mouse died a short time after the candle had been extinguished. However, he could revivify the foul air by placing green plants in the area and exposing them to light. Priestley's observations were some of the first experiments that demonstrated the activity of a photosynthetic reaction centre.
In 1779, Jan Ingenhousz carried out more than 500 experiments spread out over 4 months in an attempt to understand what was really going on. He wrote up his discoveries in a book entitled Experiments upon Vegetables. Ingenhousz took green plants and immersed them in water inside a transparent tank. He observed many bubbles rising from the surface of the leaves whenever the plants were exposed to light. Ingenhousz collected the gas that was given off by the plants and performed several different tests in attempt to determine what the gas was. The test that finally revealed the identity of the gas was placing a smouldering taper into the gas sample and having it relight. This test proved it was oxygen, or, as Joseph Priestley had called it, 'de- phlogisticated air'.
In 1932, Professor Robert Emerson and an undergraduate student, William Arnold, used a repetitive flash technique to precisely measure small quantities of oxygen evolved by chlorophyll in the algae Chlorella. Their experiment proved the existence of a photosynthetic unit. Gaffron and Wohl later interpreted the experiment and realized that the light absorbed by the photosynthetic unit was transferred. This reaction occurs at the reaction centre of photosystem II and takes place in cyanobacteria, algae and green plants.
Photosystem II
Photosystem II is the photosystem that generates the two electrons that will eventually reduce NADP+ in Ferredoxin-NADP-reduktase. Photosystem II is present on the thylakoid membranes inside chloroplasts, the site of photosynthesis in green plants. The structure of Photosystem II is remarkably similar to the bacterial reaction centre, and it is theorized that they share a common ancestor.
The core of photosystem II consists of two subunits referred to as D1 and D2. These two subunits are similar to the L and M subunits present in the bacterial reaction centre. Photosystem II differs from the bacterial reaction centre in that it has many additional subunits that bind additional chlorophylls to increase efficiency. The overall reaction catalysed by photosystem II is:
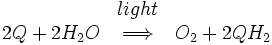
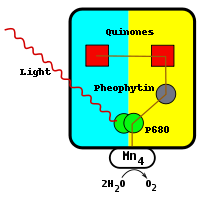
Q represents plastoquinone, the oxidized form of Q. QH2 represents plastoquinol, the reduced form of Q. This process of reducing quinone is comparable to that which takes place in the bacterial reaction centre. Photosystem II obtains electrons by oxidizing water in a process called photolysis. Molecular oxygen is a byproduct of this process, and it is this reaction that supplies the atmosphere with oxygen. The fact that the oxygen from green plants originated from water was first deduced by the Canadian-born American biochemist Martin David Kamen. He used a natural, stable isotope of oxygen, O18 to trace the path of the oxygen, from water to gaseous molecular oxygen. This reaction is catalysed by a reactive centre in photosystem II containing four manganese ions.
The reaction begins with the excitation of a pair of chlorophyll molecules similar to those in the bacterial reaction centre. Due to the presence of chlorophyll a, as opposed to bacteriochlorophyll, photosystem II absorbs light at a shorter wavelength. The pair of chlorophyll molecules at the reaction centre are often referred to as P680. When the photon has been absorbed, the resulting high-energy electron is transferred to a nearby phaeophytin molecule. This is above and to the right of the pair on the diagram and is coloured grey. The electron travels from the phaeophytin molecule through two plastoquinone molecules, the first tightly bound, the second loosely bound. The tightly bound molecule is shown above the phaeophytin molecule and is coloured red. The loosely bound molecule is to the left of this and is also coloured red. This flow of electrons is similar to that of the bacterial reaction centre. Two electrons are required to fully reduce the loosely bound plastoquinone molecule to QH2 as well as the uptake of two protons.
The difference between photosystem II and the bacterial reaction centre is the source of the electron that neutralizes the pair of chlorophyll a molecules. In the bacterial reaction centre, the electron is obtained from a reduced compound haem group in a cytochrome subunit or from a water-soluble cytochrome-c protein.
Once photoinduced charge separation has taken place, the P680 molecule carries a positive charge. P680 is a very strong oxidant and extracts electrons from two water molecules that are bound at the manganese centre directly below the pair. This centre, below and to the left of the pair in the diagram, contains four manganese ions, a calcium ion, a chloride ion, and a tyrosine residue. Manganese is efficient because it is capable of existing in four oxidation states: Mn2+, Mn3+, Mn4+ and Mn5+. Manganese also forms strong bonds with oxygen-containing molecules such as water.
Every time the P680 absorbs a photon, it emits an electron, gaining a positive charge. This charge is neutralized by the extraction of an electron from the manganese centre, which sits directly below it. The process of oxidizing two molecules of water requires four electrons. The water molecules that are oxidized in the manganese centre are the source of the electrons that reduce the two molecules of Q to QH2. To date, this water-splitting catalytic centre cannot be reproduced by any man-made catalyst.
Photosystem I
After the electron has left photosystem II it is transferred to a cytochrome b6f complex and then to plastocyanin, a blue copper protein and electron carrier. The plastocyanin complex carries the electron that will neutralize the pair in the next reaction centre, photosystem I.
As with photosystem II and the bacterial reaction centre, a pair of chlorophyll a molecules initiates photoinduced charge separation. This pair is referred to as P700. 700 Is a reference to the wavelength at which the chlorophyll molecules absorb light maximally. The P700 lies in the centre of the protein. Once photoinduced charge separation has been initiated, the electron travels down a pathway through a chlorophyll α molecule situated directly above the P700, through a quinone molecule situated directly above that, through three 4Fe-4S clusters, and finally to an interchangeable ferredoxin complex. Ferredoxin is a soluble protein containing a 2Fe-2S cluster coordinated by four cysteine residues. The positive charge left on the P700 is neutralized by the transfer of an electron from plastocyanin. Thus the overall reaction catalysed by photosystem I is:
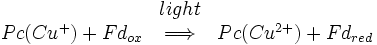
The cooperation between photosystems I and II creates an electron flow from H2O to NADP+. This pathway is called the 'Z-scheme' because the redox diagram from P680 to P700 resembles the letter z.