Dysprosium
Did you know...
SOS Children have produced a selection of wikipedia articles for schools since 2005. Sponsoring children helps children in the developing world to learn too.
| Dysprosium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
66Dy
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
silvery white |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | dysprosium, Dy, 66 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | / d ɪ s ˈ p r oʊ z i ə m / dis-PROH-zee-əm |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metallic category | lanthanide | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | n/a, 6, f | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 162.500 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
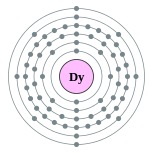
| Electron configuration | [Xe] 4f10 6s2 2, 8, 18, 28, 8, 2 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Lecoq de Boisbaudran (1886) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.540 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 8.37 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1680 K, 1407 °C, 2565 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2840 K, 2562 °C, 4653 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 11.06 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 280 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 27.7 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 3, 2, 1 (weakly basic oxide) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.22 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 573.0 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1130 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2200 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 178 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 192±7 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic at 300 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | ( r.t.) (α, poly) 926 nΩ·m | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 10.7 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | ( r.t.) (α, poly) 9.9 µm/(m·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 2710 m·s−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | (α form) 61.4 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | (α form) 24.7 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | (α form) 40.5 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | (α form) 0.247 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 540 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 500 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7429-91-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of dysprosium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Dysprosium is a chemical element with the symbol Dy and atomic number 66. It is a rare earth element with a metallic silver luster. Dysprosium is never found in nature as a free element, though it is found in various minerals, such as xenotime. Naturally occurring dysprosium is composed of 7 isotopes, the most abundant of which is 164Dy.
Dysprosium was first identified in 1886 by Paul Émile Lecoq de Boisbaudran, but was not isolated in pure form until the development of ion exchange techniques in the 1950s. Dysprosium is used for its high thermal neutron absorption cross-section in making control rods in nuclear reactors, for its high magnetic susceptibility in data storage applications, and as a component of Terfenol-D. Soluble dysprosium salts are mildly toxic, while the insoluble salts are considered non-toxic.
Characteristics
Physical properties
Dysprosium is a rare earth element that has a metallic, bright silver luster. It is soft enough to be cut with a knife, and can be machined without sparking if overheating is avoided. Dysprosium's physical characteristics can be greatly affected even by small amounts of impurities.
Dysprosium and holmium have the highest magnetic strengths of the elements, especially at low temperatures. Dysprosium has a simple ferromagnetic ordering at temperatures below 85 K (−188.2 °C). Above 85 K (−188.2 °C), it turns into an helical antiferromagnetic state in which all of the atomic moments in a particular basal plane layer are parallel, and oriented at a fixed angle to the moments of adjacent layers. This unusual antiferromagnetism transforms into a disordered ( paramagnetic) state at 179 K (−94 °C).
Chemical properties
Dysprosium metal tarnishes slowly in air and burns readily to form dysprosium(III) oxide:
- 4 Dy + 3 O2 → 2 Dy2O3
Dysprosium is quite electropositive and reacts slowly with cold water and quite quickly with hot water to form dysprosium hydroxide:
- 2 Dy (s) + 6 H2O (l) → 2 Dy(OH)3 (aq) + 3 H2 (g)
Dysprosium metal vigorously reacts with all the halogens at above 200 °C:
- 2 Dy (s) + 3 F2 (g) → 2 DyF3 (s) [green]
- 2 Dy (s) + 3 Cl2 (g) → 2 DyCl3 (s) [white]
- 2 Dy (s) + 3 Br2 (g) → 2 DyBr3 (s) [white]
- 2 Dy (s) + 3 I2 (g) → 2 DyI3 (s) [green]
Dysprosium dissolves readily in dilute sulfuric acid to form solutions containing the yellow Dy(III) ions, which exist as a [Dy(OH2)9]3+ complexes:
- 2 Dy (s) + 3 H2SO4 (aq) → 2 Dy3+ (aq) + 3 SO2−
4 (aq) + 3 H2 (g)
Compounds
Dysprosium halides, such as DyF3 and DyBr3, tend to take on a yellow colour. Dysprosium oxide, also known as dysprosia, is a white powder that is highly magnetic, more so than iron oxide.
Dysprosium combines with various non-metals at high temperatures to form binary compounds with varying composition and oxidation states +3 and sometimes +2, such as DyN, DyP, DyH2 and DyH3; DyS, DyS2, Dy2S3 and Dy5S7; DyB2, DyB4, DyB6 and DyB12, as well as Dy3C and Dy2C3.
Dysprosium carbonate, Dy2(CO3)3, and dysprosium sulfate, Dy2(SO4)3, result from similar reactions. Most dysprosium compounds are soluble in water, though dysprosium carbonate tetrahydrate (Dy2(CO3)3·4H2O) and dysprosium oxalate decahydrate (Dy2(C2O4)3·10H2O) are both insoluble in water.
Isotopes
Naturally occurring dysprosium is composed of 7 isotopes: 156Dy, 158Dy, 160Dy, 161Dy, 162Dy, 163Dy, and 164Dy. These are all considered stable, although 156Dy decays by alpha decay with a half-life of over 1×1018 years. Of the naturally occurring isotopes, 164Dy is the most abundant at 28%, followed by 162Dy at 26%. The least abundant is 156Dy at .06%.
Twenty-nine radioisotopes have also been synthesized, ranging in atomic mass from 138 to 173. The most stable of these is 154Dy with a half-life of approximately 3×106 years, followed by 159Dy with a half-life of 144.4 days. The least stable is 138Dy with a half-life of 200 ms. Isotopes that are lighter than the stable isotopes tend to decay primarily by β+ decay, while those that are heavier tend to decay by β− decay, with some exceptions. 154Dy decays primarily by alpha decay, and 152Dy and 159Dy decay primarily by electron capture. Dysprosium also has at least 11 metastable isomers, ranging in atomic mass from 140 to 165. The most stable of these is 165mDy, which has a half-life of 1.257 minutes. 149Dy has two metastable isomers, the second of which, 149m2Dy, has a half-life of 28 ns.
History
In 1878, erbium ores were found to contain the oxides of holmium and thulium. French chemist Paul Émile Lecoq de Boisbaudran, while working with holmium oxide, separated dysprosium oxide from it in Paris in 1886. His procedure for isolating the dysprosium involved dissolving dysprosium oxide in acid, then adding ammonia to precipitate the hydroxide. He was only able to isolate dysprosium from its oxide after more than 30 attempts at his procedure. Upon succeeding, he named the element dysprosium from the Greek dysprositos (δυσπρόσιτος), meaning "hard to get". However, the element was not isolated in relatively pure form until after the development of ion exchange techniques by Frank Spedding at Iowa State University in the early 1950s.
In 1950, Glenn T. Seaborg, Albert Ghiorso, and Stanley G. Thompson bombarded 241Am with helium ions, which produced atoms with an atomic number of 97 and which closely resembled the neighboring lanthanide terbium. Because terbium was named after Ytterby, the city in which it and several other elements were discovered, this new element was named berkelium for the city in which it was synthesized. However, when the research team synthesized element 98, they could not think of a good analogy for dysprosium, and instead named the element californium in honour of the state in which it was synthesized. The research team went on to "point out that, in recognition of the fact that dysprosium is named on the basis of a Greek word meaning 'difficult to get at,' that the searchers for another element a century ago found it difficult to get to California."
Occurrence
Dysprosium is never encountered as a free element, but is found in many minerals, including xenotime, fergusonite, gadolinite, euxenite, polycrase, blomstrandine, monazite and bastnäsite; often with erbium and holmium or other rare earth elements. Currently, most dysprosium is being obtained from the ion-adsorption clay ores of southern China, and future sources will include the Halls Creek region in Western Australia. In the high-yttrium version of these, dysprosium happens to be the most abundant of the heavy lanthanides, comprising up to 7–8% of the concentrate (as compared to about 65% for yttrium). The concentration of Dy in the Earth crust is about 5.2 mg/kg and in sea water 0.9 ng/L.
Production
Dysprosium is obtained primarily from monazite sand, a mixture of various phosphates. The metal is obtained as a by-product in the commercial extraction of yttrium. In isolating dysprosium, most of the unwanted metals can be removed magnetically or by a flotation process. Dysprosium can then be separated from other rare earth metals by an ion exchange displacement process. The resulting dysprosium ions can then react with either fluorine or chlorine to form dysprosium fluoride, DyF3, or dysprosium chloride, DyCl3. These compounds can be reduced using either calcium or lithium metals in the following reactions:
- 3 Ca + 2 DyF3 → 2 Dy + 3 CaF2
- 3 Li + DyCl3 → Dy + 3 LiCl
The components are placed in a tantalum crucible and fired in a helium atmosphere. As the reaction progresses, the resulting halide compounds and molten dysprosium separate due to differences in density. When the mixture cools, the dysprosium can be cut away from the impurities.
About 100 tonnes of dysprosium are produced worldwide each year, with 99% of that total produced in China. Dysprosium prices have climbed nearly twentyfold, from $7 per pound in 2003, to $130 a pound in late 2010. According to the United States Department of Energy, the wide range of its current and projected uses, together with the lack of any immediately suitable replacement, makes dysprosium the single most critical element for emerging clean energy technologies - even their most conservative projections predict a shortfall of dysprosium before 2015.
Applications
Dysprosium is used, in conjunction with vanadium and other elements, in making laser materials and commercial lighting. Because of dysprosium's high thermal neutron absorption cross-section, dysprosium oxide-nickel cermets are used in neutron-absorbing control rods in nuclear reactors. Dysprosium-cadmium chalcogenides are sources of infrared radiation which is useful for studying chemical reactions. Because dysprosium and its compounds are highly susceptible to magnetization, they are employed in various data storage applications, such as in hard disks.
Neodymium-iron-boron magnets can have up to 6% of the neodymium substituted with dysprosium to raise the coercivity for demanding applications such as drive motors for hybrid electric vehicles. This substitution would require up to 100 grams of dysprosium per hybrid car produced. Based on Toyota's projected 2 million units per year, the use of dysprosium in applications such as this would quickly exhaust the available supply of the metal. The dysprosium substitution may also be useful in other applications, as it improves the corrosion resistance of the magnets.
Dysprosium is one of the components of Terfenol-D, along with iron and terbium. Terfenol-D has the highest room-temperature magnetostriction of any known material; this property is employed in transducers, wide-band mechanical resonators, and high-precision liquid fuel injectors.
Dysprosium is used in dosimeters for measuring ionizing radiation. Crystals of calcium sulfate or calcium fluoride are doped with dysprosium. When these crystals are exposed to radiation, the dysprosium atoms become excited and luminescent. The luminescence can be measured to determine the degree of exposure to which the dosimeter has been subjected.
Nanofibers of dysprosium compounds have high strength and large surface area; therefore, they can be used for reinforcement of other materials and as a catalyst. Fibers of dysprosium oxide fluoride can be produced by heating an aqueous solution of DyBr3 and NaF to 450 °C at 450 bar pressure for 17 hours. This material is remarkably robust, surviving over 100 hours in various aqueous solutions at temperatures exceeding 400 °C without re-dissolving or aggregating.
Dysprosium iodide and dysprosium bromide are used in high intensity lighting. These compounds dissociate near the hot centre of the lamp releasing isolated dysprosium atoms. The latter re-emit light in the green and red part of the spectrum thereby effectively producing bright light.
Precautions
Like many powders, dysprosium powder may present an explosion hazard when mixed with air and when an ignition source is present. Thin foils of the substance can also be ignited by sparks or by static electricity. Dysprosium fires cannot be put out by water. It can react with water to produce flammable hydrogen gas. Dysprosium chloride fires, however, can be extinguished with water, while dysprosium fluoride and dysprosium oxide are non-flammable. Dysprosium nitrate, Dy(NO3)3, is a strong oxidizing agent and will readily ignite upon contact with organic substances.
Soluble dysprosium salts, such as dysprosium chloride and dysprosium nitrate, are mildly toxic when ingested. The insoluble salts, however, are non-toxic. Based on the toxicity of dysprosium chloride to mice, it is estimated that the ingestion of 500 grams or more could be fatal to a human.