Gallium
Background Information
SOS Children have produced a selection of wikipedia articles for schools since 2005. SOS Child sponsorship is cool!
| Gallium | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
31Ga
|
|||||||||||||||||||
|
|||||||||||||||||||
| Appearance | |||||||||||||||||||
silver-white |
|||||||||||||||||||
| General properties | |||||||||||||||||||
| Name, symbol, number | gallium, Ga, 31 | ||||||||||||||||||
| Pronunciation | / ˈ ɡ æ l i ə m / GAL-ee-əm | ||||||||||||||||||
| Element category | post-transition metal | ||||||||||||||||||
| Group, period, block | 13, 4, p | ||||||||||||||||||
| Standard atomic weight | 69.723(1) | ||||||||||||||||||
| Electron configuration | [Ar] 4s2 3d10 4p1 2, 8, 18, 3 |
||||||||||||||||||
| History | |||||||||||||||||||
| Prediction | Dmitri Mendeleev (1871) | ||||||||||||||||||
| Discovery | Lecoq de Boisbaudran (1875) | ||||||||||||||||||
| First isolation | Lecoq de Boisbaudran (1875) | ||||||||||||||||||
| Physical properties | |||||||||||||||||||
| Phase | solid | ||||||||||||||||||
| Density (near r.t.) | 5.91 g·cm−3 | ||||||||||||||||||
| Liquid density at m.p. | 6.095 g·cm−3 | ||||||||||||||||||
| Melting point | 302.9146 K, 29.7646 °C, 85.5763 °F | ||||||||||||||||||
| Boiling point | 2477 K, 2204 °C, 3999 °F | ||||||||||||||||||
| Heat of fusion | 5.59 kJ·mol−1 | ||||||||||||||||||
| Heat of vaporization | 254 kJ·mol−1 | ||||||||||||||||||
| Molar heat capacity | 25.86 J·mol−1·K−1 | ||||||||||||||||||
| Vapor pressure | |||||||||||||||||||
|
|||||||||||||||||||
| Atomic properties | |||||||||||||||||||
| Oxidation states | 3, 2, 1 ( amphoteric oxide) |
||||||||||||||||||
| Electronegativity | 1.81 (Pauling scale) | ||||||||||||||||||
| Ionization energies ( more) |
1st: 578.8 kJ·mol−1 | ||||||||||||||||||
| 2nd: 1979.3 kJ·mol−1 | |||||||||||||||||||
| 3rd: 2963 kJ·mol−1 | |||||||||||||||||||
| Atomic radius | 135 pm | ||||||||||||||||||
| Covalent radius | 122±3 pm | ||||||||||||||||||
| Van der Waals radius | 187 pm | ||||||||||||||||||
| Miscellanea | |||||||||||||||||||
| Crystal structure | orthorhombic | ||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||
| Electrical resistivity | (20 °C) 270 nΩ·m | ||||||||||||||||||
| Thermal conductivity | 40.6 W·m−1·K−1 | ||||||||||||||||||
| Thermal expansion | (25 °C) 18 µm·m−1·K−1 | ||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 2740 m·s−1 | ||||||||||||||||||
| Young's modulus | 9.8 GPa | ||||||||||||||||||
| Poisson ratio | 0.47 | ||||||||||||||||||
| Mohs hardness | 1.5 | ||||||||||||||||||
| Brinell hardness | 60 MPa | ||||||||||||||||||
| CAS registry number | 7440-55-3 | ||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||
| Main article: Isotopes of gallium | |||||||||||||||||||
|
|||||||||||||||||||
Gallium is a chemical element with symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium(III) compounds in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. Held long enough, gallium will melt in the hand as it liquefies at temperature of 29.76 °C (85.57 °F) (slightly above room temperature). Its melting point is used as a temperature reference point. The alloy Galinstan (68.5% Ga, 21.5% In, 10% Sn) has an even lower melting point of −19 °C (−2 °F), well below the freezing point of water. From its discovery in 1875 until the semiconductor era, gallium was used primarily as an agent to make low-melting alloys.
Today, almost all gallium is used for microelectronics. Gallium arsenide, the primary use of gallium, is used in microwave circuitry and infrared applications. Gallium nitride and indium gallium nitride, minority semiconductor uses, produce blue and violet light-emitting diodes (LEDs) and diode lasers.
Gallium has no known role in biology. Because gallium(III) and ferric salts behave similarly in biological systems, gallium ions often mimic iron ions in medical applications. Gallium-containing pharmaceuticals and radiopharmaceuticals have been developed.
Physical properties
Elemental gallium is not found in nature, but it is easily obtained by smelting. Very pure gallium metal has a brilliant silvery colour and its solid metal fractures conchoidally like glass. Gallium metal expands by 3.1% when it solidifies, and therefore storage in either glass or metal containers is avoided, due to the possibility of container rupture with freezing. Gallium shares the higher-density liquid state with only a few materials like silicon, germanium, bismuth and water.
Gallium attacks most other metals by diffusing into their metal lattice. Gallium, for example, diffuses into the grain boundaries of Al/Zn alloys or steel, making them very brittle. Gallium easily alloys with many metals, and is used in small quantities as a plutonium-gallium alloy in the plutonium cores of nuclear bombs, to help stabilize the plutonium crystal structure.
The melting point of 302.9146 K (29.7646 °C, 85.5763 °F) is near room temperature, about the average summer time temperatures. Gallium's melting point (mp) is one of the formal temperature reference points in the International Temperature Scale of 1990 (ITS-90) established by BIPM. The triple point of gallium of 302.9166 K (29.7666 °C, 85.5799 °F), is being used by NIST in preference to gallium's melting point.
The unique melting point of gallium allows it to melt in one's hand, and then refreeze if removed. This metal has a strong tendency to supercool below its melting point/freezing point. Seeding with a crystal helps to initiate freezing. Gallium is one of the metals (with caesium, rubidium, mercury and likely francium) that are liquid at or near-normal room temperature, and can therefore be used in metal-in-glass high-temperature thermometers. It is also notable for having one of the largest liquid ranges for a metal, and (unlike mercury) for having a low vapor pressure at high temperatures. Gallium's boiling point, 2477 K, is more than eight times higher than its melting point on the absolute scale, making it the greatest ratio between melting point and boiling point of any element. Unlike mercury, liquid gallium metal wets glass and skin, making it mechanically more difficult to handle (even though it is substantially less toxic and requires far fewer precautions). For this reason as well as the metal contamination and freezing-expansion problems, samples of gallium metal are usually supplied in polyethylene packets within other containers.
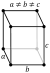
| Property | a | b | c |
|---|---|---|---|
| α (~25 °C, µm/m) | 16 | 11 | 31 |
| ρ (29.7 °C, nΩ·m) | 543 | 174 | 81 |
| ρ (0 °C, nΩ·m) | 480 | 154 | 71.6 |
| ρ (77 K, nΩ·m) | 101 | 30.8 | 14.3 |
| ρ (4.2 K, pΩ·m) | 13.8 | 6.8 | 1.6 |
Gallium does not crystallize in any of the simple crystal structures. The stable phase under normal conditions is orthorhombic with 8 atoms in the conventional unit cell. Each atom has only one nearest neighbour (at a distance of 244 pm) and six other neighbors within additional 39 pm. Many stable and metastable phases are found as function of temperature and pressure.
The bonding between the two nearest neighbors is covalent, hence Ga2 dimers are seen as the fundamental building blocks of the crystal. This explains the drop of the melting point compared to its neighbour elements aluminium and indium.
The physical properties of gallium are highly anisotropic, i.e. have different values along the three major crystallographical axes a, b and c (see table); for this reason, there is a significant difference between the linear (α) and volume thermal expansion coefficients. The properties of gallium are also strongly temperature-dependent, especially near the melting point. For example, the thermal expansion coefficient increases by several hundred percent upon melting.
Chemical properties
Gallium is found primarily in the +3 oxidation state. The +1 oxidation is also attested in some compounds, although they tend to disproportionate into elemental gallium and gallium(III) compounds. What are sometimes referred to as gallium(II) compounds are actually mixed-oxidation state compounds containing both gallium(I) and gallium(III).
Chalcogen compounds
At room temperature, gallium metal is unreactive towards air and water due to the formation of a passive, protective oxide layer. At higher temperatures, however, it reacts with oxygen in the air to form gallium(III) oxide, Ga2O3. Reducing Ga2O3 with elemental gallium in vacuum at 500 °C to 700 °C yields the dark brown gallium(I) oxide, Ga2O. Ga2O is a very strong reducing agent, capable of reducing H2SO4 to H2S. It disproportionates at 800 °C back to gallium and Ga2O3.
Gallium sulfide, Ga2S3, has 3 possible crystal modifications. It can be made by the reaction of gallium with hydrogen sulfide (H2S) at 950 °C. Alternatively, Ga(OH)3 can also be used at 747 °C:
- 2 Ga(OH)3 + 3 H2S → Ga2S3 + 6 H2O
Reacting a mixture of alkali metal carbonates and Ga2O3 with H2S leads to the formation of thiogallates containing the [Ga2S4]2− anion. Strong acids decompose these salts, releasing H2S in the process. The mercury salt, HgGa2S4, can be used as a phosphor.
Gallium also forms sulfides in lower oxidation states, such as gallium(II) sulfide and the green gallium(I) sulfide, the latter of which is produced from the former by heating to 1000 °C under a stream of nitrogen.
The other binary chalcogenides, Ga2Se3 and Ga2Te3, have zincblende structure. They are all semiconductors, but are easily hydrolysed, limiting their usefulness.
Aqueous chemistry
Strong acids dissolve gallium, forming gallium(III) salts such as Ga2(SO4)3 and Ga(NO3)3. Aqueous solutions of gallium(III) salts contain the hydrated gallium ion, [Ga(H2O)6]3+. Gallium(III) hydroxide, Ga(OH)3, may be precipitated from gallium(III) solutions by adding ammonia. Dehydrating Ga(OH)3 at 100 °C produces gallium oxide hydroxide, GaO(OH).
Alkaline hydroxide solutions dissolve gallium, forming gallate salts containing the Ga(OH)−
4 anion. Gallium hydroxide, which is amphoteric, also dissolves in alkali to form gallate salts. Although earlier work suggested Ga(OH)3−
6 as another possible gallate anion, this species was not found in later work.
Pnictogen compounds
Gallium reacts with ammonia at 1050 °C to form gallium nitride, GaN. Gallium also forms binary compounds with phosphorus, arsenic, and antimony: gallium phosphide (GaP), gallium arsenide (GaAs), and gallium antimonide (GaSb). These compounds have the same structure as ZnS, and have important semiconducting properties. GaP, GaAs, and GaSb can be synthesized by the direct reaction of gallium with elemental phosphorus, arsenic, or antimony. They exhibit higher electrical conductivity than GaN. GaP can also be synthesized by the reaction of Ga2O with phosphorus at low temperatures.
Gallium also forms ternary nitrides; for example:
- Li3Ga + N2 → Li3GaN2
Similar compounds with phosphorus and arsenic also exist: Li3GaP2 and Li3GaAs2. These compounds are easily hydrolyzed by dilute acids and water.
Halides
Gallium(III) oxide reacts with fluorinating agents such as HF or F2 to form gallium(III) fluoride, GaF3. It is an ionic compound strongly insoluble in water. However, it does dissolve in hydrofluoric acid, in which it forms an adduct with water, GaF3·3H2O. Attempting to dehydrate this adduct instead forms GaF2OH·nH2O. The adduct reacts with ammonia to form GaF3·3NH3, which can then be heated to form anhydrous GaF3.
Gallium trichloride is formed by the reaction of gallium metal with chlorine gas. Unlike the trifluoride, gallium(III) chloride exists as dimeric molecules, Ga2Cl6, with a melting point of 78 °C. This is also the case for the bromide and iodide, Ga2Br6 and Ga2I6.
Like the other group 13 trihalides, gallium(III) halides are Lewis acids, reacting as halide acceptors with alkali metal halides to form salts containing GaX−
4 anions, where X is a halogen. They also react with alkyl halides to form carbocations and GaX−
4.
When heated to a high temperature, gallium(III) halides react with elemental gallium to form the respective gallium(I) halides. For example, GaCl3 reacts with Ga to form GaCl:
- 2 Ga + GaCl3
 3 GaCl (g)
3 GaCl (g)
At lower temperatures, the equilibrium shifts toward the left and GaCl disproportionates back to elemental gallium and GaCl3. GaCl can also be made by the reaction of Ga with HCl at 950 °C; it can then be condensed as red solid.
Gallium(I) compounds can be stabilized by forming adducts with Lewis acids. For example:
- GaCl + AlCl3 → Ga+[AlCl4]−
The so-called "gallium(II) halides", GaX2, are actually adducts of gallium(I) halides with the respective gallium(III) halides, having the structure Ga+[GaX4]−. For example:
- GaCl + GaCl3 → Ga+[GaCl4]−
Hydrogen compounds
Like aluminium, gallium also forms a hydride, GaH3, known as gallane, which may be obtained by the reaction of lithium gallanate (LiGaH4) with gallium(III) chloride at −30 °C:
- 3 LiGaH4 + GaCl3 → 3 LiCl + 4 GaH3
In the presence of dimethyl ether as solvent, GaH3 polymerizes to (GaH3)n. If no solvent is used, the dimer Ga2H6 ( digallane) is formed as a gas. Its structure is similar to diborane, having two hydrogen atoms bridging the two gallium centers, unlike α- AlH3 in which aluminium has a coordination number of 6.
Gallane is unstable above −10 °C, decomposing to elemental gallium and hydrogen.
History
In 1871, existence of gallium was first predicted by Russian chemist Dmitri Mendeleev, who named it " eka-aluminium" on the basis of its position in his periodic table. He also predicted several properties of the element, which correspond closely to real gallium properties, such as density, melting point, oxide character and bonding in chloride.
Gallium was discovered spectroscopically by French chemist Paul Emile Lecoq de Boisbaudran in 1875 by its characteristic spectrum (two violet lines) in an examination of a sphalerite sample. Later that year, Lecoq obtained the free metal by electrolysis of its hydroxide in potassium hydroxide solution. He named the element "gallia", from Latin Gallia meaning Gaul, after his native land of France. It was later claimed that, in one of those multilingual puns so beloved of men of science in the 19th century, he had also named gallium after himself, as his name, "Le coq", is the French for "the rooster", and the Latin for "rooster" is "gallus"; however, in an 1877 article Lecoq denied this supposition. (Cf. the naming of the J/ψ meson and the dwarf planet Pluto.)
From its discovery in 1875 up to the era of semiconductors, its primary uses were in high-temperature thermometric applications and in preparation of metal alloys with unusual properties of stability, or ease of melting; some being liquid at room temperature or below. The development of gallium arsenide as a direct band gap semiconductor in the 1960s ushered in the most important stage in the applications of gallium.
Occurrence
Gallium does not exist in free form in nature, and the few high-gallium minerals such as gallite (CuGaS2) are too rare to serve as a primary source of the element or its compounds. Its abundance in the Earth's crust is approximately 16.9 ppm. Gallium is found and extracted as a trace component in bauxite and to a small extent from sphalerite. The amount extracted from coal, diaspore and germanite in which gallium is also present is negligible. The United States Geological Survey ( USGS) estimates gallium reserves to exceed 1 million tonnes, based on 50 ppm by weight concentration in known reserves of bauxite and zinc ores. Some flue dusts from burning coal have been shown to contain small quantities of gallium, typically less than 1% by weight.
Production
Gallium is a byproduct of the production of aluminium and zinc. Whereas the sphalerite for zinc production is the minor source. Most gallium is extracted from the crude aluminium hydroxide solution of the Bayer process for producing alumina and aluminium. A mercury cell electrolysis and hydrolysis of the amalgam with sodium hydroxide leads to sodium gallate. Electrolysis then gives gallium metal. For semiconductor use, further purification is carried out using zone melting, or else single crystal extraction from a melt ( Czochralski process). Purities of 99.9999% are routinely achieved and commercially widely available.
In 1986, the production was estimated at 40 tons. In 2007 the production of gallium was 184 tonnes with less than 100 tonnes from mining and the rest from scrap recycling. By 2011 world production of gallium was an estimated 216 metric tons.
Applications
The semiconductor applications dominate the commercial use of gallium, accounting for 98% of applications. The next major application is for gadolinium gallium garnets.
Semiconductors
Because of this application, extremely high-purity (99.9999+%) gallium is commercially available. Gallium arsenide (GaAs) and gallium nitride (GaN) used in electronic components represented about 98% of the gallium consumption in the United States in 2007. About 66% of semiconductor gallium is used in the U.S. in integrated circuits (mostly gallium arsenide), such as the manufacture of ultra-high speed logic chips and MESFETs for low-noise microwave preamplifiers in cell phones. About 20% is used in optoelectronics. Worldwide, gallium arsenide makes up 95% of the annual global gallium consumption.
Gallium arsenide is used in optoelectronics in a variety of infrared applications. Aluminium gallium arsenide (AlGaAs) is used in high-powered infrared laser diodes. As a component of the semiconductors indium gallium nitride and gallium nitride, gallium is used to produce blue and violet optoelectronic devices, mostly laser diodes and light-emitting diodes. For example, gallium nitride 405 nm diode lasers are used as a violet light source for higher-density compact disc data storage, in the Blu-ray Disc standard.
Multijunction photovoltaic cells, developed for satellite power applications, are made by molecular beam epitaxy or metalorganic vapour phase epitaxy of thin films of gallium arsenide, indium gallium phosphide or indium gallium arsenide.The Mars Exploration Rovers and several satellites use triple junction gallium arsenide on germanium cells. Gallium is also a component in photovoltaic compounds (such as copper indium gallium selenium sulfide or Cu(In,Ga)(Se,S)2) for use in solar panels as a cost-efficient alternative to crystalline silicon.
Galinstan and other alloys
Gallium readily alloys with most metals, and has been used as a component in low-melting alloys. A nearly eutectic alloy of gallium, indium, and tin is a room temperature liquid that is available in medical thermometers. This alloy, with the trade-name Galinstan (with the "-stan" referring to the tin), has a low freezing point of −19 °C (−2.2 °F). It has been suggested that this family of alloys could also be used to cool computer chips in place of water. Gallium alloys have been evaluated as substitutes for mercury dental amalgams, but these materials have yet to see wide acceptance.
Because gallium wets glass or porcelain, gallium can be used to create brilliant mirrors. When the wetting action of gallium-alloys is not desired (as in Galinstan glass thermometers), the glass must be protected with a transparent layer of gallium(III) oxide.
The plutonium used in nuclear weapon pits is machined by alloying with gallium to stabilize its δ phase.
Gallium added in quantities up to 2% in common solders can aid wetting and flow characteristics.
Alloys of Al and Ga have been evaluated for hydrogen production.
It is used as alloying element in the magnetic shape-memory alloy Ni-Mn-Ga.
Biomedical applications
Although gallium has no known role in biology, it mimics iron(III), the gallium ion localizes to and interacts with many processes in the body in which iron(III) is manipulated. As these processes include inflammation, which is a marker for many disease states, several gallium salts are used, or are in development, as both pharmaceuticals and radiopharmaceuticals in medicine. When gallium ions are mistakenly taken up by bacteria such as Pseudomonas, the bacteria's ability to respire is interfered with and the bacteria die. The mechanism behind this is that iron is redox active, which allows for the transfer of electrons during respiration, but gallium is redox inactive.
Gallium nitrate (brand name Ganite) has been used as an intravenous pharmaceutical to treat hypercalcemia associated with tumor metastasis to bones. Gallium is thought to interfere with osteoclast function. It may be effective when other treatments for maligancy-associated hypercalcemia are not.
- Gallium maltolate, an orally absorbable form of gallium(III) ion, is in clinical and preclinical trials as a potential treatment for a number of types of cancer, infectious disease, and inflammatory disease.
A complex amine-phenol Ga(III) compound MR045 was found to be selectively toxic to parasites that have developed resistance to chloroquine, a common drug against malaria. Both the Ga(III) complex and chloroquine act by inhibiting crystallization of hemozoin, a disposal product formed from the digestion of blood by the parasites.
Radiogallium salts
Gallium-67 salts such as gallium citrate and gallium nitrate are used as radiopharmaceutical agents in a nuclear medicine imaging procedure commonly referred to as a gallium scan. The form or salt of gallium is unimportant. For these applications, the radioactive isotope 67Ga is used. The body handles Ga3+ in many ways as though it were iron, and thus it is bound (and concentrates) in areas of inflammation, such as infection, and also areas of rapid cell division. This allows such sites to be imaged by nuclear scan techniques. This use has largely been replaced by fluorodeoxyglucose (FDG) for positron emission tomography, "PET" scan and indium-111 labelled leukocyte scans. However, the localization of gallium in the body has some properties which make it unique in some circumstances from competing modalities using other radioisotopes.
Gallium-68, a positron emitter with a half life of 68 min., is now used as a diagnostic radionuclide in PET-CT when linked to pharmaceutical preparations such as DOTATOC, a somatostatin analogue used for neuroendocrine tumors investigation, and DOTA-TATE, a newer one, used for neuroendocrine metastasis and lung neuroendocrine cancer, such as certain types of microcytoma. Galium-68's preparation as a pharmaceutical is chemical and the radionuclide is extracted by elution from germanium-68, a synthetic radioisotope of germanium, in gallium-68 generators.
Other uses
- Magnesium gallate containing impurities (such as Mn2+), is beginning to be used in ultraviolet-activated phosphor powder.
- Neutrino detection. Possibly the largest amount of pure gallium ever collected in a single spot is the Gallium-Germanium Neutrino Telescope used by the SAGE experiment at the Baksan Neutrino Observatory in Russia. This detector contains 55–57 tonnes of liquid gallium. Another experiment was the GALLEX neutrino detector operated in the early 1990s in an Italian mountain tunnel. The detector contained 12.2 tons of watered gallium-71. Solar neutrinos caused a few atoms of 71Ga to become radioactive 71Ge, which were detected. The solar neutrino flux deduced was found to have a deficit of 40% from theory. This was not explained until better solar neutrino detectors and theories were constructed (see SNO).
- As a liquid metal ion source for a focused ion beam. For example, a focused-gallium-ion beam was used to create the world's smallest book, Teeny Ted from Turnip Town.
- In a classic prank, scientists would fashion gallium spoons and serve tea to unsuspecting guests. The spoons melt in the hot tea.
- As an additive in glide wax for skiis, and other low friction surface materials. US 5069803, Sugimura, Kentaro; Shoji Hasimoto & Takayuki Ono, "Use of a synthetic resin composition containing gallium particles in the glide surfacing material of skis and other applications", issued 1995
Precautions
The Ga(III) ion of soluble gallium salts tends to form the insoluble hydroxide when injected in large amounts, and in animals precipitation of this has resulted in renal toxicity. In lower doses, soluble gallium is tolerated well, and does not accumulate as a poison.
While metallic gallium is not considered toxic, the data are inconclusive. Some sources suggest that it may cause dermatitis from prolonged exposure; other tests have not caused a positive reaction. Like most metals, finely divided gallium loses its luster and powdered gallium appears gray. Thus, when gallium is handled with bare hands, the extremely fine dispersion of liquid gallium droplets, which results from wetting skin with the metal, may appear as a gray skin stain.